SIGVARIS is a Medical
Equipment
Manufacturing group based in the Peachtree City, GA USA. Sigvaris offers high quality and
innovative medical compression solutions that improve health and well being.
Manufacturing
As SIGVARIS GROUP, we are a reliable and experienced partner to our customers around the
world which include Pharmacies, DME, Orthopedic and Medical Specialist stores as well as
Medical Professionals and Hospitals. They advise individuals and provide them with products
and solutions for medical compression.

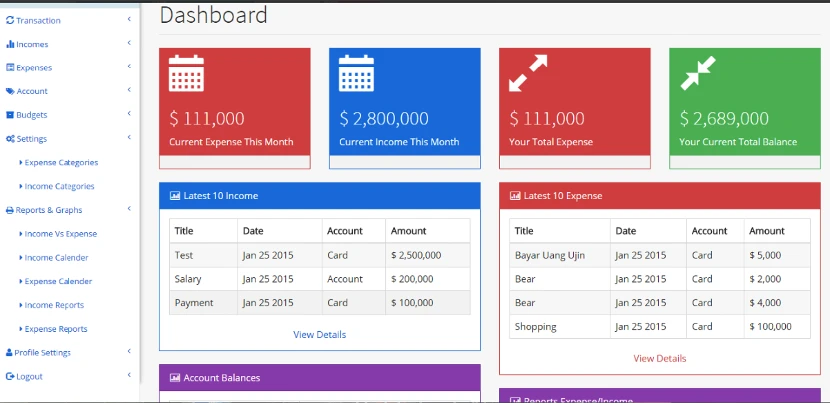
As the previous
system was
outdated, and difficult to use. SIGVARIS GROUP was looking to build a CRM system software
with modern technology to manage the sales process, track customers and manage their order
history. Streamline the sales process by easily identifying business opportunities and new
leads.
As the previous
system was difficult to use, when the DigiPrima Expert team began the search for a
replacement system, the most important factor was the simplicity of the new system.